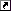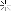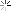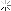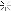-
Contributing Member


Nickle/Chrome Stripping Question
I successfully stripped chrome /nickle plating from a project rifle. When the chrome and nickle came off the copper wash from the plating process remains.
How can I get it off? Looking for suggestions. The bright spots are where the metal was affected by corrosion and the bare steel is through in some areas. Thanks, Brian
Attachment 90509
Information
 |
Warning: This is a relatively older thread
This discussion is older than 360 days. Some information contained in it may no longer be current. |
|
-
The Following 2 Members Say Thank You to Doco overboard For This Useful Post:
-
02-04-2018 07:50 PM
# ADS
Friends and Sponsors

-
Advisory Panel


I suggest you try one of the bore solvents that are sold for removing lead and copper in barrels. What I find over here appears to be strongly ammoniac-based. So don't dunk the receiver in the stuff - it could promote rusting in hard-to-get-at corners.
Wear rubber gloves, moisten a rough cloth with the solvent, and rub away on an inconspicuous patch. If the cloth starts turning blue, then it is indeed removing the copper. Carry on through a quiet hour or so, until the copper is all washed off. But do NOT forget to clean the entire receiver afterwards to remove any traces of solvent, and oil it - to prevent rust starting on the "bone-dry" steel.
-
The Following 2 Members Say Thank You to Patrick Chadwick For This Useful Post:
-
-
Advisory Panel



Originally Posted by
Doco overboard

How can I get it off?
We removed chrome from a batch of 1911 frames that came in two decades ago with silica glass beads and it took all of it off, chrome and copper all at the same time. It doesn't damage or remove features like sand blasting and you can see the color of the metal changing in the lights of the cabinet as you go. This would work for the remains here. You need to do that anyway to prepare for your blue next...
-
The Following 3 Members Say Thank You to browningautorifle For This Useful Post:
-
Contributing Member


We removed chrome from a batch of 1911 frames that came in two decades ago with silica glass beads and it took all of it off, chrome and copper all at the same time. It doesn't damage or remove features like sand blasting and you can see the color of the metal changing in the lights of the cabinet as you go. This would work for the remains here. You need to do that anyway to prepare for your blue next...
I tried ammonia based bore solvent like was suggested and made some progress. It was alot of work, some areas are more difficult than others. I want to try some glass beads but I'm unsure if I need a specific size or diameter. Or does it matter with glass?
Thanks everyone for the replies.
-
-
Advisory Panel


Silica sand glass beads are different grades I think but I forget what we used. They disappear to dust and a fine grade would work nicely... You'll watch at an angle in the light and you'll see a different shade of silver appearing... If in doubt try some cold blue for patches. You can glass bead it right back off.
-
-
Contributing Member


Thanks, I'm going to give glass beading a try. I think the cu is actually thicker than the chrome in spots.
-
-
Advisory Panel



Originally Posted by
Doco overboard

I'm going to give glass beading a try.
Look for the change in color, you'll see. Use the angle of the light reflecting to show you...it turns from a gold(ish) to a clean silver...
-
-
Legacy Member

This is what I use. Probably the same that Jim was talking about.
Best Regards.....Frank
Attachment 90573
-
-
Advisory Panel


Probably about the same, ours came in bags.
-
-
Contributing Member


Glass beads knock it right off. It would have been better to just do that right from the start because all the cu would have been removed while it was still attached to the nickle and chrome. Separating the metals has just introduced more labor into the process. There is some slight copper wash that has remained but household ammonia and some bore cleaner will work that off. A quick polish on a muslin wheel would probably get me where I need to be once all that has been done.
-











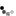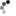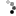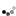



 PM
PM